Synopsis
At a conference last year, India’s new drug controller general Rajeev Raghuvanshi, who was then the scientific director at the Indian Pharmacopeia Commission, cited examples of drugs found to be less effective than desired. Having worked in the industry, he knows its shortcomings. But amid pressure from lobbyists and state agencies that follow different standards, can he make effective changes?
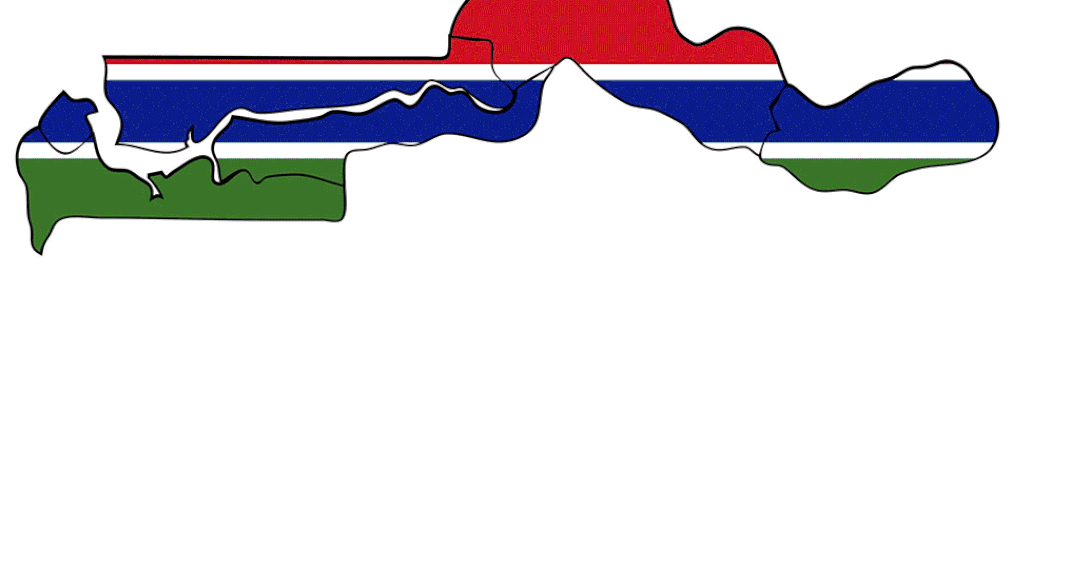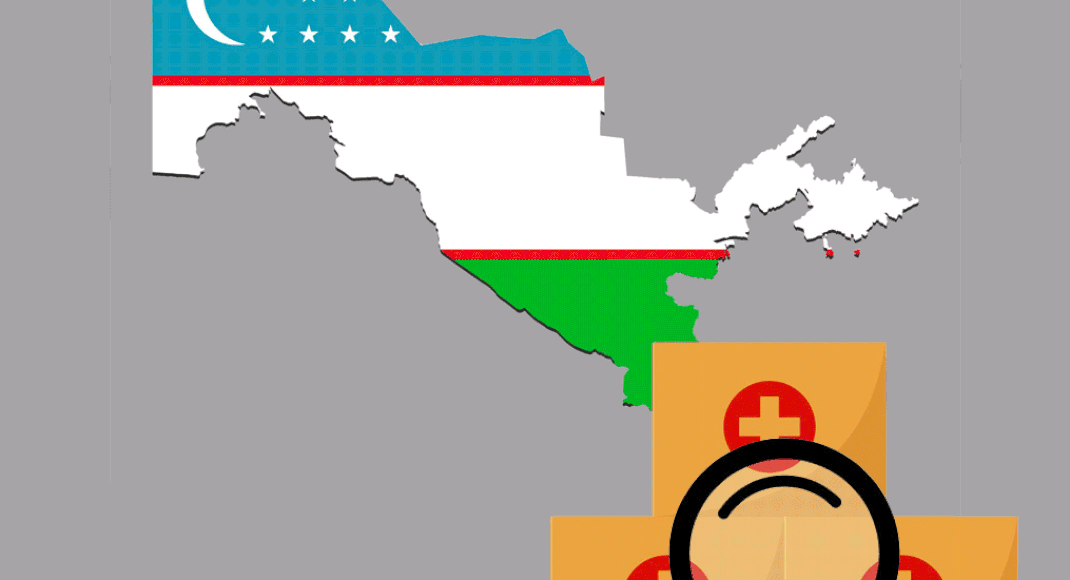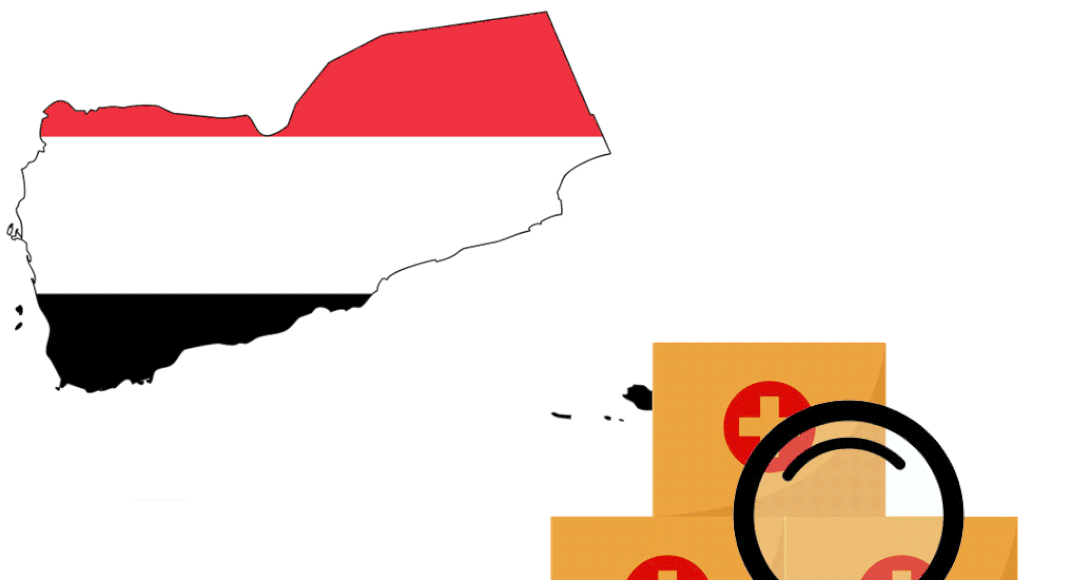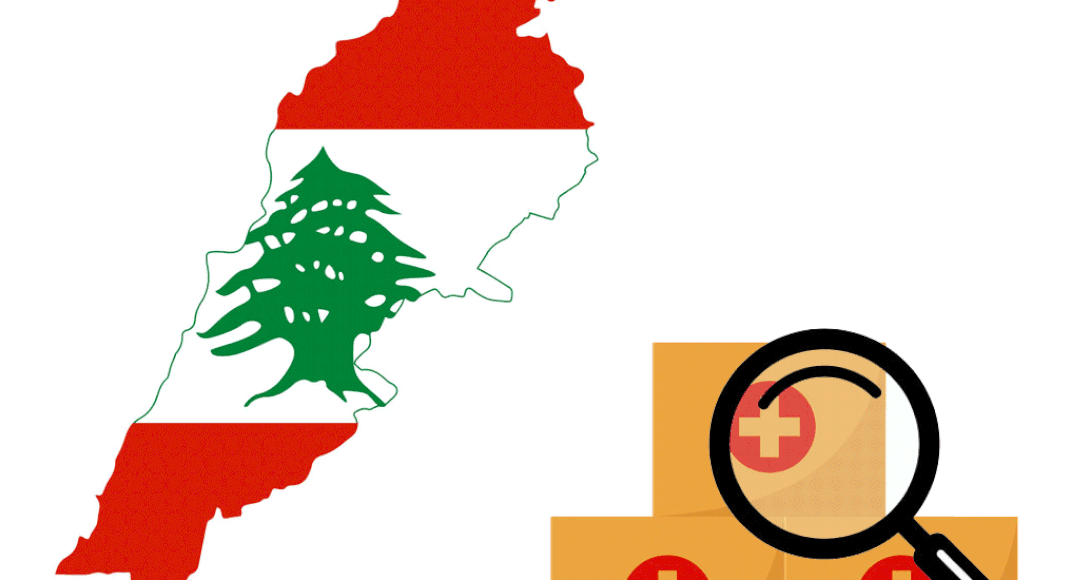
Last July, about 70 children in the small African nation of the Gambia died from acute kidney damage after having a cough syrup. Evidence, highlighted by the World Health Organization (WHO) three months later, showed the medicine supplied by Maiden Pharma, an Indian company, contained high levels of toxic impurities. The incident was a big blot on India’s reputation as a medicine exporter to low-income countries. Worse was in store. Soon after,
- FONT SIZE
AbcSmall
AbcMedium
AbcLarge
Uh-oh! This is an exclusive story available for selected readers only.
Worry not. You’re just a step away.
Why ?
Exclusive Economic Times Stories, Editorials & Expert opinion across 20+ sectors

Stock analysis. Market Research. Industry Trends on 4000+ Stocks

Clean experience with
Minimal Ads
Comment & Engage with ET Prime community 
Exclusive invites to Virtual Events with Industry Leaders 
A trusted team of Journalists & Analysts who can best filter signal from noise 
Get 1 Year Complimentary Subscription of TOI+ worth Rs.799/-